Answer:
P(0<x<
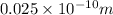 ) = 0.25
) = 0.25
Given:
l = d = 0.1 nm
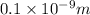
Solution:
The wave function for the confined electron in the range 0<x<
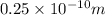 is given by:
is given by:
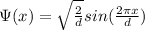
Now, the probability to find the electron in the range 0<x<
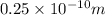 is given by:
is given by:
P(x) =
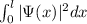
P(x) =
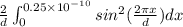
P(x) =
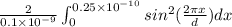
P(x) =
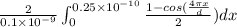
P(x) =
![\frac{2}{0.1* 10{- 9}}[(0.025* 10^(- 9)* 4\pi - (sin(4\pi* 0.025* 10^(- 9)))/(0.1* 10^(- 9)))/(8\pi)]](https://img.qammunity.org/2020/formulas/physics/college/bfb639rjdrzs1g7mxs7ftjsust1qg1bw8f.png)
On solving the above eqn, we get:
P(x) = 0.25
P(x) = 0.25