Step-by-step explanation:
The given data is as follows.
For HCl : Volume = 100 mL, Density = 1.00 g/ml, Molarity = 0.25 M
For NaOH : Volume = 200 mL, Density = 1.00 g/ml, Molarity = 0.150 M
Therefore, mass of HCl will be calculated as follows.
Density =

1.00 g/ml =
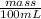
mass = 100 g
Also, number of moles =
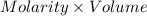
Hence, no. of moles of HCl =
 (as 1 ml = 1000 L)
(as 1 ml = 1000 L)
=
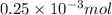
On the other hand, mass of NaOH will be calculated as follows.
Density =

1.00 g/ml =
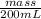
mass = 200 g
Also, number of moles =
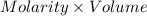
Hence, no. of moles of HCl =
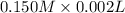 (as 1 ml = 1000 L)
(as 1 ml = 1000 L)
=
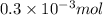
Total mass = (100 g + 200 g) = 300 g
Since, HCl is the limiting reagent over here. So, heat produced by
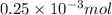 will be calculated as follows.
will be calculated as follows.
Q =
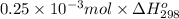
=
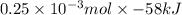
=
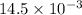 kJ
kJ
=
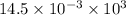 J
J
Also, it is known that relation between Q and temperature change is:
Q =
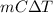
Hence, putting the values into the above formula as follows.
Q =
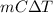
14.5 J =
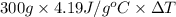
 =
=
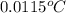
Thus, we can conclude that increase in temperature is
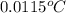 .
.