Step-by-step explanation:
1) 1.0 ounce serving of the cereal provides 130 calories.
Then 32 ounces of breakfast cereal will provide :
=
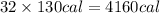
Cost of 32 ounces of cereal = $4.23
Energy from cereal with respect to dollar:
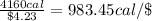
2) Density of iso-octane = d = 0.6916 g/mL
Mass of the iso-octane = m
Volume of iso-octane = V = 1 L
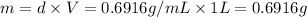
Moles of isooctane =
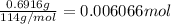
Heat of combustion of isooctane = 5450 kJ/mol
Heat released on combustion of 0.006066 moles of isooctane:
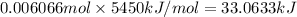
1 kJ = 239.006 cal
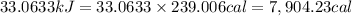
Cost of 0.6916 g or 1 L of isooctane = $0.45
Energy from isooctane with respect to dollar:
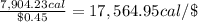
We can generate large amount of energy by combustion of isooctane than the energy we getting from cereal:
17,564.95 cal/$ > 983.45 cal/$
But isooctane is an indigestible by human body so, it will be good to have cereals.