Answer: The pH of the solution in the beginning is 1.89 and the pH of the solution after the addition of base is
Step-by-step explanation:
To calculate the pH of the solution, we use the equation:
![pH=-\log[H^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/vz65x0ueuj8r8ibqa81zvsbzb2yaetlce4.png)
We are given:
Nitric acid is a monoprotic acid and it dissociates 1 mole of hydrogen ions. So, the concentration of hydrogen ions is 0.013 M
![[H^+]=0.013M](https://img.qammunity.org/2020/formulas/chemistry/college/e28vjp9h9t9szn4wpj41u4qen8b2qy2z92.png)
Putting values in above equation, we get:
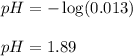
To calculate the number of moles, we use the equation:
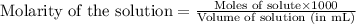
Molarity of nitric acid solution = 0.013 M
Volume of solution = 15 mL
Putting values in above equation, we get:
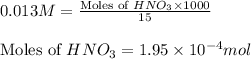
Molarity of methylamine solution = 0.017 M
Volume of solution = 10 mL
Putting values in above equation, we get:
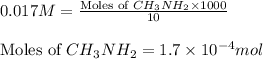
- The chemical equation for the reaction of nitric acid and methylamine follows:
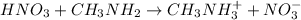
As, the mole ratio of nitric acid and methyl amine is 1 : 1. So, the limiting reagent will be the reactant whose number of moles are less, which is methyl amine.
By Stoichiometry of the reaction:
1 mole of methyl amine produces 1 mole of
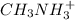
So,
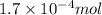 of methyl amine will produce =
of methyl amine will produce =
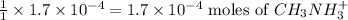
To calculate the
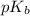 of base, we use the equation:
of base, we use the equation:
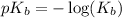
where,
 = base dissociation constant =
= base dissociation constant =
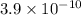
Putting values in above equation, we get:
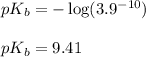
- To calculate the pOH of basic buffer, we use the equation given by Henderson Hasselbalch:
![pOH=pK_b+\log(([salt])/([base]))](https://img.qammunity.org/2020/formulas/chemistry/college/sczgwwurez8svwaa6wk2y4tedmhd42roec.png)
![pOH=pK_b+\log(([CH_3NH_3^+])/([CH_3NH_2]))](https://img.qammunity.org/2020/formulas/chemistry/college/5yoqmzb3e2q43pubg4zzo1cq2m8eztuf1v.png)
We are given:
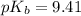
![[CH_3NH_3^+]=(1.7* 10^(-4))/(10+15)=6.8* 10^(-6)M](https://img.qammunity.org/2020/formulas/chemistry/college/eb6ps8zfcc5dfm4074jjz7dqkca42z8j0a.png)
![[CH_3NH_2]=(1.7* 10^(-4))/(10+15)=6.8* 10^(-6)M](https://img.qammunity.org/2020/formulas/chemistry/college/1rchx35ns863r7blqt4rq2chwfplvnji2l.png)
Putting values in above equation, we get:
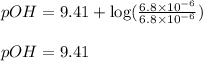
To calculate pH of the solution, we use the equation:
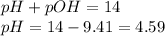
Hence, the pH of the solution is 4.59