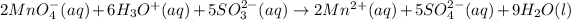Answer : The balance ionic equation for a redox reaction will be,
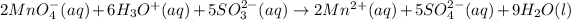
Explanation :
The half balanced redox reactions in acidic medium will be:
(1)
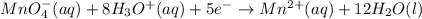
(2)
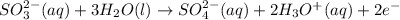
In order of balance the electrons, we multiply the equation 1 by 2 and equation 2 by 5, we get:
(1)
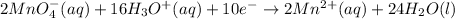
(2)
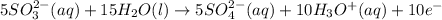
Now adding both the equation, we get:
The overall reaction will be,