Answer:
B) 8 T.
Step-by-step explanation:
For an ideal gas , the gas law that it follow is as follows
P₁V₁ / T₁ = P₂ V₂ /T₂
This law is followed by all the gases whether mono atomic or diatomic.
In this case following information are given
P₂ / P₁ = 3
V₂ / V₁ = 3
T₁ = T
T₂ = ?
From the gas law formula given , we have
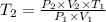
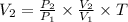
= 3 X 3 T
= 9 T
Change in temperature = 9T - T = 8 T.