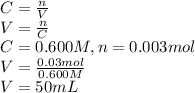Answer:
50 mL of HCl is required to react completely with 2.50g of sodium hydrogen carbonate.
Step-by-step explanation:
The balanced equation shows that 1 mol of NaHCO₃ reacts with 1 mol of HCl. Therefore, we need to calculate the amount of moles present in 2.50g of NaHCO₃ to know how much in moles we will need of HCl. Then, we need to use the concentration to calculate the volume of the HCl solution that will be required.
First step: Calculating the number of moles of NaHCO₃
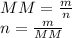
MM NaHCO₃ = 84.0 g/mol, m = 2.50 g
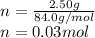
Second step: Calculating the volume of HCl