Answer:
100.8 °C
Step-by-step explanation:
The Clausius-clapeyron equation is:
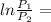 -Δ
-Δ
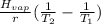
Where 'ΔHvap' is the enthalpy of vaporization; 'R' is the molar gas constant (8.314 j/mol); 'T1' is the temperature at the pressure 'P1' and 'T2' is the temperature at the pressure 'P2'
Isolating for T2 gives:
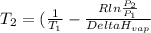
(sorry for 'deltaHvap' I can not input symbols into equations)
thus T2=100.8 °C