Answer: The mass of MgO formed is 3.502 grams and mass of
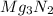 formed is 1.587 grams.
formed is 1.587 grams.
Step-by-step explanation:
We are given:
Total mass of magnesium reacted = 3.26 g
Total mass of products formed = 5.09 g
To calculate number of moles of a substance, we use the equation:
 .....(1)
.....(1)
- For calculating mass of MgO:
The chemical equation for the formation of magnesium oxide follows:
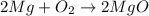
Let 'x' grams of magnesium is reacting to form magnesium oxide.
Molar mass of Magnesium = 24.3 g/mol
Putting values in equation 1, we get:
Number of moles of magnesium =
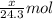
By Stoichiometry of the reaction:
2 moles of magnesium produces 2 moles of MgO
So,
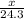 moles of magnesium will produce
moles of magnesium will produce
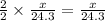 moles of MgO
moles of MgO
Molar mass of MgO = 40.3 g/mol
Putting values in equation 1, we get:
Mass of MgO =
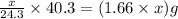
Mass of magnesium oxide =
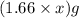 = X grams
= X grams
- For calculating mass of
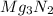 :
:
The chemical equation for the formation of magnesium nitride follows:
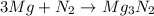
Mass of magnesium reacting = (3.26 - x)
Molar mass of Magnesium = 24.3 g/mol
Putting values in equation 1, we get:
Number of moles of magnesium =
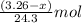
By Stoichiometry of the reaction:
3 moles of magnesium produces 1 moles of
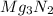
So,
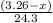 moles of magnesium will produce
moles of magnesium will produce
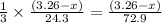 moles of
moles of
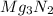
Molar mass of
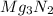 = 101 g/mol
= 101 g/mol
Putting values in equation 1, we get:
Mass of
![Mg_3N_2=((3.26-x))/(72.9)* 101=[(3.26-x)* 1.38]g](https://img.qammunity.org/2020/formulas/chemistry/high-school/wt4kfzykdu60v7tarms0eo73ialkgiqyv9.png)
Mass of magnesium nitride =
![[(3.26-x)* 1.38]g](https://img.qammunity.org/2020/formulas/chemistry/high-school/f0q27n0yv8ar6us6xcvciam8m344fpp4yb.png) = Y grams
= Y grams
- Calculating the mass of products:
Total mass of the products = 5.09 grams
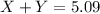
Putting values of 'X' and 'Y' in above equation, we get:
![(1.66* x)+[(3.26-x)* 1.38]=5.09\\\\x=2.11g](https://img.qammunity.org/2020/formulas/chemistry/high-school/7nqay6fsigidqkc26wj2zonsftwzm9134u.png)
Mass of MgO =
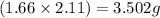
Mass of
![Mg_3N_2=[(3.26-2.11)* 1.38]=1.587g](https://img.qammunity.org/2020/formulas/chemistry/high-school/hsqzgk3iavcpu7lfgyznxoxlly2o386omu.png)
Hence, the mass of MgO formed is 3.502 grams and mass of
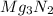 formed is 1.587 grams.
formed is 1.587 grams.