Answer:
92.04%
Step-by-step explanation:
Given:
Mass of CO₂ obtained = 53.0 grams
Mass of calcium carbonate heated = 1.31 grams
Now,
the molar mass of the calcium carbonate = 100.08 grams
The number of moles heated in the problem = Mass / Molar mass
= (1.31 grams) / (100.08 grams/moles)
= 0.013088 moles
now,
1 mol of calcium carbonate yields 1 mol of CO₂
thus,
0.013088 moles of calcium carbonate will yield = 0.013088 mol of CO₂
now,
Theoretical mass of 0.013088 moles of CO₂ will be
= Number of moles × Molar mass of CO₂
= 0.013088 × 44 = 0.5758 grams
Thus, the percent yield for this reaction =
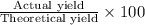
or
the percent yield for this reaction =
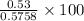
or
the percent yield for this reaction = 92.04%