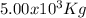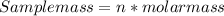Answer:
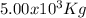
Step-by-step explanation:
There are two reactions taking place and the product of the first is a reactant at the other, so writing down the balanced chemical equations is the first step in determinimg the proportions
1) C + O2 → CO2
1. Molar mass C = 12.0107 g/mol
2. Number of moles
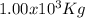 of carbon has
of carbon has
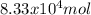 of C
of C
Remember: mol(n)
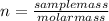
2) CO2 + 2NH3 → CO(NH3)2 + H2O
1. Molar mass CO(NH3)2 = 60.02 g/mol
2. Proportions the urea syntheres has a proportion of 1:1 with carbon dioxyde, so there is
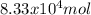 of CO(NH3)2
of CO(NH3)2
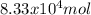 of CO(NH3)2 has
of CO(NH3)2 has