Answer: 2588 grams of ethane are there in 100 L of this gas at 80 degree celsius and 25 atm.
Step-by-step explanation:
According to the ideal gas equation:'

P = Pressure of the gas = 25 atm
V= Volume of the gas = 100 L
T= Temperature of the gas = 80°C = (80+273)K=353 K (0°C = 273 K)
R= Gas constant = 0.0821 atmL/K mol
n= moles of gas= ?
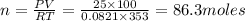
Mass of gas=
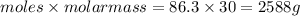
Thus 2588 grams of ethane are there in 100 L of this gas at 80 degree celsius and 25 atm.