Answer:
8.1107 g
Step-by-step explanation:
The given reaction:
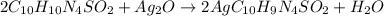
Given that:
Mass of silver sulfadiazine = 25.0 g
Molar mass of silver sulfadiazine = 357.14 g/mol
The formula for the calculation of moles is shown below:

Thus,

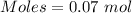
From the reaction,
2 moles of silver sulfadiazine are formed from 1 mole of silver oxide
So,
1 mole of silver sulfadiazine are formed from 1/2 mole of silver oxide
0.07 mole of silver sulfadiazine are formed from 1/2*0.07 mole of silver oxide
Moles of silver oxide = 0.035 moles
Molar mass of silver oxide = 231.735 g/mol
Mass = Moles * Molar mass = 0.035 moles * 231.735 g/mol = 8.1107 g