Answer : The concentration of
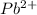 ion is 0.0375 M.
ion is 0.0375 M.
Explanation :
The balanced equilibrium reaction will be:
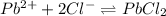
The expression for solubility constant for this reaction will be,
![K_(sp)=[Pb^(2+)][Cl^-]^2](https://img.qammunity.org/2020/formulas/mathematics/middle-school/s56gnrs0y8ewj88mg6jv3o3h9j4kofa4p9.png)
Now put all the given values in this expression, we get:
![2.40* 10^(-4)=[Pb^(2+)]* (0.0800)^2](https://img.qammunity.org/2020/formulas/chemistry/college/dnk71vwkfk7uc3azvismp1yijdjcvgjgb1.png)
![[Pb^(2+)]=0.0375M](https://img.qammunity.org/2020/formulas/chemistry/college/50xzlt97nu8415u8wrt2cchm0ixj9asdd2.png)
Therefore, the concentration of
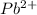 ion is 0.0375 M.
ion is 0.0375 M.