Answer:
162g per mol of octane
Step-by-step explanation:
The combustion reaction of octane is:
2C8H18 + 25O2 ⇒ 16CO2 + 18H2O
That means the reaction produces 18mol of water per 2 mol of octane burned, so if we burn 1 mol of octane we can obtain the mass of water like this:
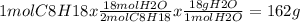 of water per mol of C8H18 burned
of water per mol of C8H18 burned