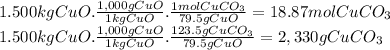Answer:
(a) 0.22 mol Cl₂ and 15.4g Cl₂
(b) 2.89.10⁻³ mol O₂ and 0.092g O₂
(c) 8 mol NaNO₃ and 680g NaNO₃
(d) 1,666 mol CO₂ and 73,333 g CO₂
(e) 18.87 CuCO₃ and 2,330g CuCO₃
Step-by-step explanation:
In most stoichiometry problems there are a few steps that we always need to follow.
- Step 1: Write the balanced equation
- Step 2: Establish the theoretical relationship between the kind of information we have and the one we are looking for. Those relationships can be found in the balanced equation.
- Step 3: Apply conversion factor/s to the data provided in the task based on the relationships we found in the previous step.
(a)
Step 1:
2 Na + Cl₂ ⇄ 2 NaCl
Step 2:
In the balanced equation there are 2 moles of Na, thus 2 x 23g = 46g of Na. 46g of Na react with 1 mol of Cl₂. Since the molar mass of Cl₂ is 71g/mol, then 46g of Na react with 71g of Cl₂.
Step 3:
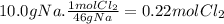
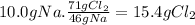
(b)
Step 1:
HgO ⇄ Hg + 0.5 O₂
Step 2:
216.5g of HgO form 0.5 moles of O₂. 216.5g of HgO form 16g of O₂.
Step 3:
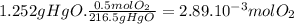
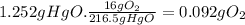
(c)
Step 1:
NaNO₃ ⇄ NaNO₂ + 0.5 O₂
Step 2:
16g of O₂ come from 1 mol of NaNO₃. 16g of O₂ come from 85g of NaNO₃.
Step 3:
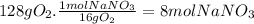
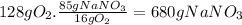
(d)
Step 1:
C + O₂ ⇄ CO₂
Step 2:
12 g of C form 1 mol of CO₂. 12 g of C form 44g of CO₂.
Step 3:
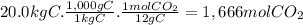
![[tex]20.0kgC.(1,000gC)/(1kgC) .(44gCO_(2))/(12gC) =73,333gCO_{2](https://img.qammunity.org/2020/formulas/chemistry/college/9yhfxpp4prlcrobburl5pmfhmu4fwf9vnx.png) [/tex]
[/tex]
(e)
Step 1:
CuCO₃ ⇄ CuO + CO₂
Step 2:
79.5g of CuO come from 1 mol of CuCO₃. 79.5g of CuO come from 123.5g of CuCO₃.
Step 3: