Step-by-step explanation:
As we are given pure water. This means that concentration of hydrogen and hydroxide ions will be the same as pure water is neutral in nature.
Also, value of
 =
=
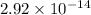 .
.
Relation between
 and concentration of hydrogen and hydroxide ions is as follows.
and concentration of hydrogen and hydroxide ions is as follows.
![K_(w) = [H^(+)][OH^(-)]](https://img.qammunity.org/2020/formulas/chemistry/college/mvyj77duld27q3ktsh21ugym0unkkr3diz.png)
Since,
![[H^(+)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/axnvi9gez4h3rovfp1qidd16ya8d7gzbon.png) =
=
![[OH^(-)]](https://img.qammunity.org/2020/formulas/chemistry/college/ug5fh4frhjslztec5ab506a86sstd1ikl9.png) .
.
So,
![K_(w) = [H^(+)][OH^(-)]](https://img.qammunity.org/2020/formulas/chemistry/college/mvyj77duld27q3ktsh21ugym0unkkr3diz.png) =
=
![[H^(+)]^(2)](https://img.qammunity.org/2020/formulas/chemistry/college/1c9nwtzgmcm38kw65vp6uj4qnqharsay8u.png)
![[H^(+)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/axnvi9gez4h3rovfp1qidd16ya8d7gzbon.png) =
=
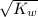
=

=

As we know that pH =
![-log[H^(+)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/pclsb5d5ztpswphl1czegwokhxu5hzr3m7.png)
pH =
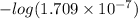
= 6.676
Thus, we can conclude that pH of pure water under given conditions is 6.676.