Answer:
![[Ni^(2+) ]=3.31x10^(-13) M](https://img.qammunity.org/2020/formulas/chemistry/college/qiq0eg26psh0nb96drd5tqh10llj8y5u6q.png)
Therefore there is
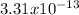 moles of
moles of
![[Ni^(2+) ]](https://img.qammunity.org/2020/formulas/chemistry/college/mpq64ystbdblnq0xs4x6nzykt7rn7f13ny.png)
in one liter of this solution.
Step-by-step explanation:
This is a problem of common ion effect. In an equilibrium, by the Le Chatelier's principle, when the concentration of a given species rises the equilibrium shifts to the direction that consumes the species that increased in concentration.
Therefore, if a common ion is present in a solubility equilibrium the molar solubility shall decrease.
1) First of all, calculate Ksp and molar solubility in pure solvent
The problem states that pKsp=15.8. The 'p' is the '-log' function, so 'pKsp' expresses '-log(Ksp)'. Isolating for 'Ksp' gives:
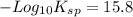
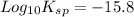
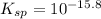
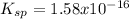
- Molar solubility in pure solvent
![K_(sp)=[Ni^(2+)][OH^(-)]^(2)](https://img.qammunity.org/2020/formulas/chemistry/college/9r5ccsri8g8yv24f1czeu4ud5sxbeuxzuh.png)
![S=[Ni^(2+)]](https://img.qammunity.org/2020/formulas/chemistry/college/nixjuxd07har9xnkvw5sy0yek7trbwht81.png) and
and
![[OH^(-)]=2S](https://img.qammunity.org/2020/formulas/chemistry/college/l3y2f8c7umeh0pl0zio0gomdeal5eca6xq.png) , where 'S' stands for molar solubility.
, where 'S' stands for molar solubility.
Then Ksp expression can be expressed as:
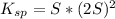
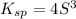
Isolating for 'S' gives us the molar solubility in pure water:
![S=\sqrt[3]{(K_(sp) )/(4) }](https://img.qammunity.org/2020/formulas/chemistry/college/dxg4enhbpvuji6agq9fur4ukn2x0zkw2eu.png)
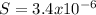
We only calculate the pure solvent molar solubility to compare with the solubility with the common ion and make sure the first is grater, witch confirms the Le Chatelier's principle.
2) Calculate the molar solubility with the common ion
- First we calculate the
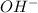 concentration from the pH value
concentration from the pH value
In a solution with a pH of 12.34 the pOH is 1.66 (14=pH+pOH)
![-Log_(10) [OH^(-) ]=1.66](https://img.qammunity.org/2020/formulas/chemistry/college/v3159oljg55imftiqbws8tnlrdtuc8x5a3.png)
![Log_(10) [OH^(-) ]=-1.66](https://img.qammunity.org/2020/formulas/chemistry/college/vk194su6uiir5zpylloch0seyl0jsozsv4.png)
![[OH^(-) ]=10^(-1.66)](https://img.qammunity.org/2020/formulas/chemistry/college/ngajwzn7140qn6xch93kc8ff2p3gfnlkec.png)
![[OH^(-) ]=0.0219](https://img.qammunity.org/2020/formulas/chemistry/college/jmu61can311hju6n17f9guzpn9lv05mpbx.png)
- Then we input this value in the Ksp expression
![K_(sp)=[Ni^(2+)][OH^(-)]^(2)](https://img.qammunity.org/2020/formulas/chemistry/college/9r5ccsri8g8yv24f1czeu4ud5sxbeuxzuh.png)
![[Ni^(2+)]=(K_(sp))/([OH^(-)]^(2))](https://img.qammunity.org/2020/formulas/chemistry/college/7fxq03j9laxqmvrut0sytsuv086ugxnpsw.png)
![[Ni^(2+)]=(1.58x10^(-16))/(0.0219^(2))](https://img.qammunity.org/2020/formulas/chemistry/college/rcr2h9n2syn0fbc3fxoqrm5b20uf49gybb.png)
![[Ni^(2+)]=3.31x10^(-13)](https://img.qammunity.org/2020/formulas/chemistry/college/2zdh942pnnhsv3u5f74o2nlgsvskhavhis.png)
The molar solubility of Ni(OH)2 when there is excess OH- in solution is lower than when the solvent is pure, which indicates that the Le Chatelier's principle has been obeyed.