Answer:
a) ε = 6.86x
 J
J
b) ε/Kavg = 10.98
Step-by-step explanation:
a) The heat of evaporation (Lv) is
Lv = εn
ε = Lv/n
n is the number of molecules in a gram of the substance. The molar mass of water (H2O) is 2x1 g of H + 16 g of O = 18 g/mol.
By the Avogadros' number(NA), we know that 1 mol = 6.02x
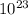 molecules, so n will be:
molecules, so n will be:
n = NA/ molar mass
n =
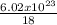
n = 3.34x
 molecules/g
molecules/g
So,
ε =
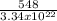
ε = 1.64x
 cal
cal
As 1 cal = 4.18 J, ε = 6.86x
 J
J
b) For ideal gases, the average of the kinetic energy (the internal energy of the molecules) is given by :
Kavg =
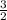 kT
kT
Where Kavg is the average of the kinetic energy, k is the Boltzmann's constant and T is the temperature in Kelvin.
Knowing that k = 1.38x
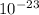 J/K and T = 29.2ºC + 273 = 302.2 K
J/K and T = 29.2ºC + 273 = 302.2 K
Kavg =
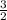 x1.38x
x1.38x
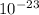 x302.2
x302.2
Kavg = 6.25x
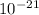 J
J
So the ratio of ε to the average kinetic energy of H2O molecules is
ε/Kavg =

ε/Kavg = 10.98