Step-by-step explanation:
(a)
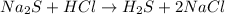
This is acid base reaction because there is no change of oxidation state on either side of the reaction.
(b)
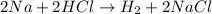
This is a oxidation reduction reaction because sodium in elemental state ( 0 oxidation state) oxidizes to Na⁺ in NaCl. Also H⁺ in HCl reduces to H° in H₂.
(c)
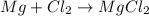
This is a oxidation reduction reaction because magnesium in elemental state ( 0 oxidation state) oxidizes to Mg²⁺ in MgCl₂. Also Cl° in Cl₂ reduces to Cl⁻ in MgCl₂.
(d)
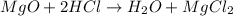
This is acid base reaction because there is no change of oxidation state on either side of the reaction.
(e)
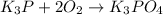
This is a oxidation reduction reaction because phosphorous in P³⁻ in K₃P oxidizes to P⁵⁺ in K₃PO₄ and oxygen reduces.
(f)
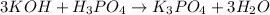
This is acid base reaction because there is no change of oxidation state on either side of the reaction.