Step-by-step explanation:
Oxidation reduction reactions are the reactions in which one specie oxidizes and other reduces.
For example,
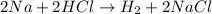
This is a oxidation reduction reaction because sodium in elemental state ( 0 oxidation state) oxidizes to Na⁺ in NaCl. Also H⁺ in HCl reduces to H° in H₂.
Elements are present in 0 oxidation state and either it is formed or consumed, its oxidation state has to be changed for a chemical reaction to occur. Hence, it is true.