Explanation :
Redox reaction or Oxidation-reduction reaction : It is defined as the reaction in which the oxidation and reduction reaction takes place simultaneously.
Oxidation reaction : It is defined as the reaction in which a substance looses its electrons. In this, oxidation state of an element increases. Or we can say that in oxidation, the loss of electrons takes place.
Reduction reaction : It is defined as the reaction in which a substance gains electrons. In this, oxidation state of an element decreases. Or we can say that in reduction, the gain of electrons takes place.
(a) The balanced chemical reaction will be:
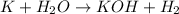
The oxidation-reduction half reaction will be :
Oxidation :
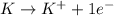
Reduction :
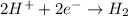
In this reaction, the oxidation state of 'K' changes from (0) to (+1) that means 'K' lost 1 electron and it shows oxidation and the oxidation state of 'H' changes from (+1) to (0) that means 'H' gains 1 electron and it shows reduction.
In this reaction, potassium is the oxidizing atom that has the highest oxidation state is (+1).
(b) The balanced chemical reaction will be:
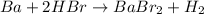
The oxidation-reduction half reaction will be :
Oxidation :
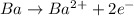
Reduction :
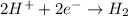
In this reaction, the oxidation state of 'Ba' changes from (0) to (+2) that means 'Ba' lost 2 electrons and it shows oxidation and the oxidation state of 'H' changes from (+1) to (0) that means 'H' gains 1 electron and it shows reduction.
In this reaction, barium is the oxidizing atom that has the highest oxidation state is (+2).
(c) The balanced chemical reaction will be:
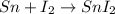
The oxidation-reduction half reaction will be :
Oxidation :
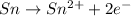
Reduction :

In this reaction, the oxidation state of 'Sn' changes from (0) to (+2) that means 'Sn' lost 2 electron and it shows oxidation and the oxidation state of 'I' changes from (0) to (-1) that means 'I' gains 1 electron and it shows reduction.
In this reaction, tin is the oxidizing atom that has the highest oxidation state is (+2).