Step-by-step explanation:
a)

oxidation state of H = +1
Oxidation state of O = -2
Let the oxidation no. of S be x.
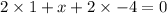
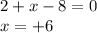
Oxidation state of S in
 = +6
= +6
b)
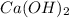
oxidation state of H = +1
Oxidation state of O = -2
Let the oxidation no. of Ca be x.

Oxidation state of Ca in
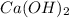 = +2
= +2
c) BrOH
oxidation state of H = +1
Oxidation state of O = -2
Let the oxidation no. of Br be x.
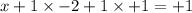
Oxidation no. of Br in BrOH is +1
d)
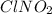
oxidation state of Cl = -1
Oxidation state of O = -2
Let the oxidation no. of N be x.
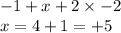
Oxidation state of N in
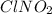 = +3
= +3
e)
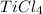
oxidation state of Cl = -1
Let the oxidation no. of Ti be x.
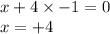
Oxidation state of Ti in
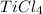 = +4
= +4
f) NaH
Na is more electropositive than H.
Therefore, oxidation state of Na = +1
Oxidation state of H = -1