Answer:
For a: The reaction is a type of synthesis reaction.
For b: The reaction is a type of double displacement reaction
For c: The reaction is a type of combustion reaction.
Step-by-step explanation:
The given chemical reaction is:
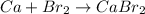
The above reaction is a type of synthesis reaction because calcium and bromine are combining in their elemental state to form a calcium bromide compound.
The given chemical reaction is:
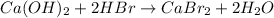
The above reaction is a type of double displacement reaction because here exchange of ions takes place.
The given chemical reaction is:
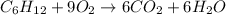
The above reaction is a type of combustion reaction because a hydrocarbon is reacting with oxygen gas to produce carbon dioxide and water molecule.