Step-by-step explanation:
Reaction between chlorine and hydrogen gas will take place as follows.
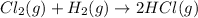
Since, standard enthalpy of
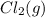 is 0 kJ/mol and
is 0 kJ/mol and
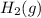 is also 0 kJ/mol.
is also 0 kJ/mol.
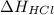 is -92.30 kJ/mol.
is -92.30 kJ/mol.
Hence, change in enthalpy will be calculated as follows.
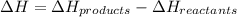
= (-92.30 - 0) kJ/mol
= -ve
Also, formation of bonds is taking place between both chlorine and hydrogen atoms. As a result, heat will be released during this process.
Thus, we can conclude that change in enthalpy will be negative.