Answer:
375 g
Step-by-step explanation:
Let the total mass of the solution = x g
Given that the mass of NaOH in the solution = 15.0 g
Thus, mass of the solvent = x - 15.0 g
Given that the mass % of the solution = 4 %
So,
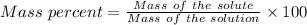
So,
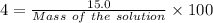
Mass of the solution = 375 g