Answer: The molarity of HCl solution is 0.12 M
Step-by-step explanation:
To calculate the molarity of solution, we use the equation:
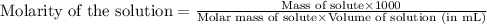
We are given:
Given mass of HCl = 868.8 g
Molar mass of HCl = 36.5 g/mol
Volume of the solution = 200 L
Putting values in above equation, we get:
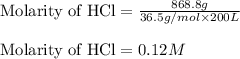
Hence, the molarity of HCl solution is 0.12 M.