Answer: B) Zinc will act as Anode
Step-by-step explanation:
Standard reduction potential of zinc and nickel are:
![E^0_([Zn^(2+)/Zn])= -0.76V](https://img.qammunity.org/2020/formulas/chemistry/college/92kagvev0ox41l5zf6f3hrus121uooywpn.png)
![E^0_([Ni^(2+)/Ni])=-0.25V](https://img.qammunity.org/2020/formulas/physics/college/htbuui1xluevl0gv5nikvdy29jfa9xkzuz.png)
Here Zinc undergoes oxidation by loss of electrons, thus act as anode as it has more negative reduction potential.
Nickel undergoes reduction by gain of electrons and thus act as cathode. as it has less negative reduction potential.
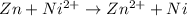
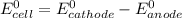
Where both
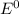 are standard reduction potentials.
are standard reduction potentials.
![E^0=E^0_([Ni^(2+)/Ni])- E^0_([Zn^(2+)/Zn])](https://img.qammunity.org/2020/formulas/physics/college/70wvvwadzubx3h8abkf4xiibemvwtdfhid.png)
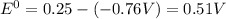
As the emf is positive, the reaction is spontaneous and reaction will occur.
Thus Zinc will act as anode.