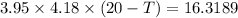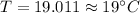Answer:
Step-by-step explanation:
Given
mass of water
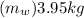
Temperature of water is
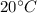
mass of ice
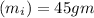
Temperature of ice
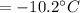
specific heat of water
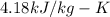
specific heat of ice
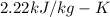
Latent heat of fusion(L)
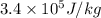
Heat absorb by water when ice completely converts to water
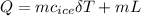
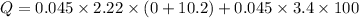
Q=1.01898+15.3=16.3189 KJ
thus temperature of water will be