Answer:
42.11 g
Step-by-step explanation:
Mass of the hydrated salt = 60 g
The moles of the hydrated salt is :
Amount = 60 g
Molar mass of
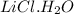 = 60.4093 g/mol
= 60.4093 g/mol
The formula for the calculation of moles is shown below:
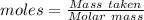
Thus, moles are:
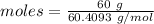
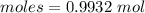
After heating only LiCl remains in the crucible. So,
Moles of LiCl = 0.9932 moles
Molar mass of
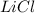 = 42.394 g/mol
= 42.394 g/mol
The formula for the calculation of moles is shown below:

Also, mass is
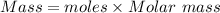
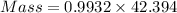
Total mass of LiCl remaining in the crucible = 42.11 g