Answer:
Such molecule must have molecular formula of C15N3H15
Step-by-step explanation:
Mass of carbon in such molecule
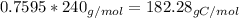
The atomic mass of carbon is 12.01 g/mol, so in 182.28 g of carbon there is 15.18 mols of carbon.
Mass of Nitrogen in such molecule
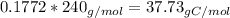
The atomic mass of nitrogen is 14.01 g/mol, so in 42.53g of nitrogen there is 3.04 mols of nitrogen.
Mass of Hydrogen in such molecule
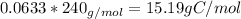
The atomic mass of Hydrogen is 1.00 g/mol, so in 15.19 g of Hydrogen there is 15.19 mols of Hydrogen.
Such molecule must have molecular formula of C15N3H15