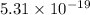Answer:
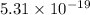
Step-by-step explanation:
Entropy of the system,
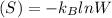
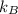 is Boltzmann constant, W is the number of microstates
is Boltzmann constant, W is the number of microstates
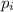 is the probability of a molecule in a given energy level.
is the probability of a molecule in a given energy level.
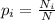
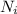 = number of molecules in a energy state, N = total number of molecules
= number of molecules in a energy state, N = total number of molecules
And,
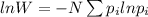
In the given problem
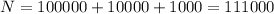
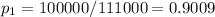
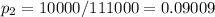
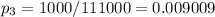
then,
![S = - N k_(B) [p_(1) lnp_(1) + p_(2)lnp_(2) + p_(2)lnp_(2)]](https://img.qammunity.org/2020/formulas/physics/college/2fzidwmzbgshv0i65s7w003s2l8s2ytw98.png)
Therefore,
![S = - Nk_(B) [ 0.9009ln0.9009 + 0.09009ln0.09009 + 0.009009ln0.009]\\S== -111000* 1.38*10^(-23)[ - 0.9009* 0.1 - 0.09009* 2.41 - 0.009009* 4.71]\\S= 111* 1.38* 10^(-20) [ 0.09009 + 0.217+0.04243]\\S= 151.8* 10^(-20)* 0.34952\\S=53.06* 10^(-20) JK^(-1) \\S=5.31* 10^(-19) JK^(-1)](https://img.qammunity.org/2020/formulas/physics/college/c66r8dk25l8287194n4jb7gpelv9uekxbt.png)
Therefore entropy of the system is