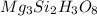Answer:
The molecular formula of chyrsotile asbestos :
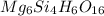
The empirical formula for chyrsotile asbestos :
Step-by-step explanation:
The molar mass of chyrsotile = M= 520.8 g/mol.
Let the molecular formula of chyrsotile be
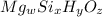
Percentage of Mg = 28.03%
Percentage of Si =21.60%
Percentage of H=1.16%
Percentage of O = 49.21%
Percentage of an element in a compound:
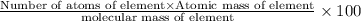
1) Magnesium atom with mass 24 g/mol.
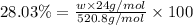
w = 6.0
2) Silicon atom with mass 28 g/mol.
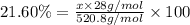
x = 4.0
3) Hydrogen atom with mass 1 g/mol.
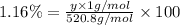
y = 6.0
4) Oxygen atom with mass 28 g/mol.
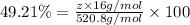
z = 16.0
The molecular formula of chyrsotile asbestos :
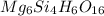
Empirical is the lowest ratio of numbers of different atoms present in a compound.
The empirical formula for chyrsotile asbestos :