Answer:
633 nm
Step-by-step explanation:
E = Energy difference = 1.96 eV
c = Speed of light = 3×10⁸ m/s
h = Planck's constant = 6.626×10⁻³⁴ J/s
Converting eV to J
1 eV = 1.6×10⁻¹⁹ J
1.96 eV = 1.96×1.6×10⁻¹⁹ Joule = 3.136×10⁻¹⁹ Joule
Photon energy equation
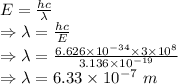
∴ Wavelength of light emitted by this laser is 633 nm