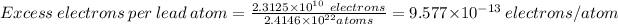Answer:
(a)
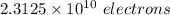
(b)
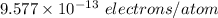
Step-by-step explanation:
(a) Let the number of the excess electrons on sphere is x.
The charge of the electron (e) =
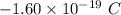 .
.
Given, the net charge (q) =
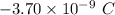
Number of excess electrons is:
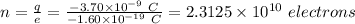
(b) Given that the mass of the sphere = 8.30 g
Molar mass = 207 g/mol
Since, 1 mole of Pb contains 6.022×10²³ atoms of Pb
Also, 1 mole mass = 207 g
207 g of Pb contains 6.022×10²³ atoms of Pb
1 g of Pb contains 6.022×10²³ / 207 atoms of Pb
So,
8.30 g of Pb contains (6.022×10²³ / 207)*8.30 atoms of Pb
No of atoms of Pb in 8.30g = 2.4146×10²² atoms