Answer:
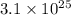 carbon atoms are present in stone
carbon atoms are present in stone
Step-by-step explanation:
Diamond is made of carbon only.
1 mg = 0.001 g
1 carat = 200 mg
So, weight of diamond in grams =
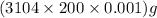 = 620.8 g
= 620.8 g
Molar mass of carbon = 12 g/mol
So, number of moles of carbon in diamond is the ratio of diamond to molar mass of carbon.
So, number of moles of carbon in diamond =
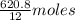
1 mol of an atom =
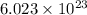 number of atoms
number of atoms
So,
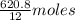 of carbon atom =
of carbon atom =
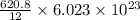 carbon atoms =
carbon atoms =
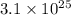 carbon atoms.
carbon atoms.