Answer : The
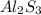 contains the greatest mass of aluminum.
contains the greatest mass of aluminum.
Explanation :
For
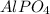 :
:
Molar mass of
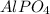 = 122 g/mole
= 122 g/mole
Molar mass of aluminium = 27 g/mole
As, 122 g of
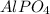 contains 27 g of Al
contains 27 g of Al
So, 122 g of
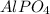 contains
contains
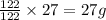 of Al
of Al
The mass of 'Al' in
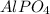 is, 27 grams.
is, 27 grams.
For
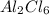 :
:
Molar mass of
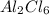 = 266.7 g/mole
= 266.7 g/mole
Molar mass of aluminium = 27 g/mole
As, 266.7 g of
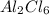 contains
contains
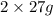 of Al
of Al
So, 266 g of
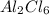 contains
contains
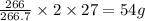 of Al
of Al
The mass of 'Al' in
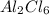 is, 54 grams.
is, 54 grams.
For
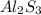 :
:
Molar mass of
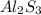 = 150 g/mole
= 150 g/mole
Molar mass of aluminium = 27 g/mole
As, 150 g of
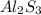 contains
contains
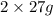 of Al
of Al
So, 225 g of
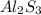 contains
contains
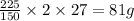 of Al
of Al
The mass of 'Al' in
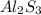 is, 81 grams.
is, 81 grams.
Hence, from this we conclude that
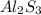 contains the greatest mass of aluminum.
contains the greatest mass of aluminum.