Answer:
442.3 mL
Step-by-step explanation:
Remember that Molarity is a measure of concentration in Chemistry and it's defined as the number of moles of the substance divided by liters of the solution:
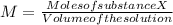
Then, you can express 11.27 g of AgNO3 as moles of AgNO3 using the molar mass of the compound:
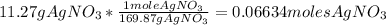
Then you can solve for the volume of the solution:
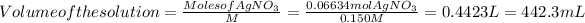
Hope it helps!