Answer: 1 mole of methane has the greatest mass of hydrogen.
Step-by-step explanation:
To calculate the mass of hydrogen, we use the equation:
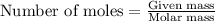 .....(1)
.....(1)
- For 1 mole of
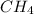
In 1 mole of methane, 1 mole of carbon atom and 4 moles of hydrogen atoms are there.
Molar mass of hydrogen = 1 g/mol
Moles of hydrogen = 4 moles
Putting values in equation 1, we get:
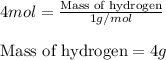
The mass of hydrogen in 1 mole of
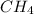 is 4 g
is 4 g
- For 0.6 mole of
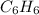
In 1 mole of benzene, 6 moles of carbon atoms and 6 moles of hydrogen atoms are there.
So, in 0.6 moles of benzene, (6 × 0.6) = 3.6 moles of carbon atoms and (6 × 0.6) = 3.6 moles of hydrogen atoms are there.
Molar mass of hydrogen = 1 g/mol
Moles of hydrogen = 3.6 moles
Putting values in equation 1, we get:
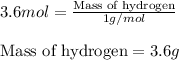
The mass of hydrogen in 0.6 mole of
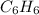 is 3.6 g
is 3.6 g
- For 0.4 mole of

In 1 mole of
 , 3 moles of carbon atom and 8 moles of hydrogen atoms are there.
, 3 moles of carbon atom and 8 moles of hydrogen atoms are there.
So, in 0.4 moles of
 , (3 × 0.4) = 1.2 moles of carbon atoms and (8 × 0.4) = 3.2 moles of hydrogen atoms are there.
, (3 × 0.4) = 1.2 moles of carbon atoms and (8 × 0.4) = 3.2 moles of hydrogen atoms are there.
Molar mass of hydrogen = 1 g/mol
Moles of hydrogen = 3.2 moles
Putting values in equation 1, we get:
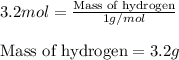
The mass of hydrogen in 0.4 mole of
 is 3.2 g
is 3.2 g
Hence, 1 mole of methane has the greatest mass of hydrogen.