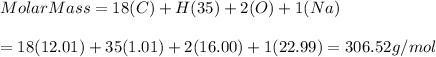Answer:
a)C2HBrClF3 = 197.35 g/mol
b)C12H14N2CL2 = 229.06g/mol
c)C8H10N4O2 = 194.22g/mol
d) CO(NH2)2=60.07 g/mol
e)C17H35CO2Na = 306.52 g/mol
Step-by-step explanation:
Molar mass of a compound is equal to the sum of the atomic masses of the constituent elements.
a) C2HBrClF3
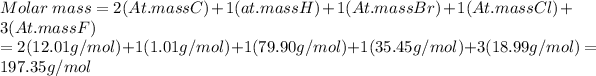
b) C12H14N2CL2
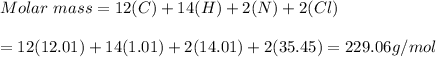
c) C8H10N4O2
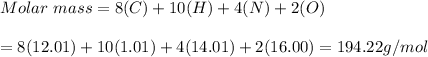
d) CO(NH2)2
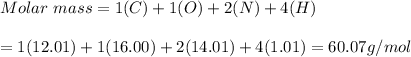
e) C17H35CO2Na