Step-by-step explanation:
Calcium chloride is an ionic compound as it is formed by transfer of an electron to each chlorine atom.
So, being an ionic compound calcium chloride is able to dissociate completely into water.
Hence, the dissociation reaction will be as follows.
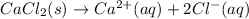
Since, two electrons has been lost by single calcium atom. Therefore, calcium atom will have a charge of +2.
Thus, we can conclude that the charge on the calcium ion, in elementary units is +2.