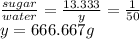Answer:
The resulting solution contains approximately 666 g of water.
Step-by-step explanation:
In the initial solution we have:
1g salt : 8g sugar : 200g water
This means that the ratios are:
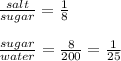
In the final solution we have:
5g salt: xg sugar: yg water
The new ratios are:
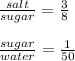
Now we can calculate the amount of sugar in the final solution:
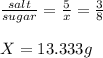
Finally, we calculate the amount of water: