Answer:
formic acid
Step-by-step explanation:
(a) 0.75 moles of
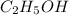
1 mole of
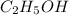 contains 1 mole of O
contains 1 mole of O
so,
0.75 moles of
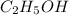 contains 0.75 mole of O
contains 0.75 mole of O
Molar mass of O = 16 g/mol
Mass = moles* Molar mass = 0.75 moles × 16 g/mol = 12 g
(b) 0.60 moles of
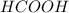
1 mole of
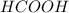 contains 2 moles of O
contains 2 moles of O
so,
0.60 moles of
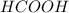 contains 0.60*2 mole of O
contains 0.60*2 mole of O
Moles of O = 1.2
Molar mass of O = 16 g/mol
Mass = moles* Molar mass = 1.2 moles × 16 g/mol = 19.2 g
(c) 1 mole of

1 mole of
 contains 1 mole of O
contains 1 mole of O
Molar mass of O = 16 g/mol
Mass = moles* Molar mass = 1 mole × 16 g/mol = 16 g
Greatest mass = 19.2 g = formic acid.