Step-by-step explanation:
(a) As per the mole concept, one mole of any atom contains
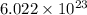 atoms or molecules, that is, Avogadro's number of atoms.
atoms or molecules, that is, Avogadro's number of atoms.
Therefore, 1 mole of
 =
=
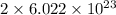 molecules
molecules
=
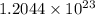 molecules
molecules
1 mole of
 =
=
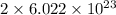 molecules
molecules
=
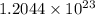 molecules
molecules
1 mole of
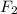 =
=
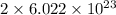 molecules
molecules
=
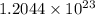 molecules
molecules
Hence, there are equal number of molecules present in the given atoms.
(b) Mass of each given atom will be calculated as follows.
Mass = no. of moles × molar mass
As one molecule of
 contains 2 atoms of hydrogen.
contains 2 atoms of hydrogen.
So, mass of 1 mole of
 =
=

= 2.016 g
mass of 1 mole of
 =
=
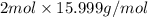
= 31.996 g
mass of 1 mole of
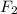 =
=
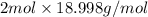
= 37.996 g
Thus, we can conclude that
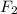 has the greatest mass.
has the greatest mass.