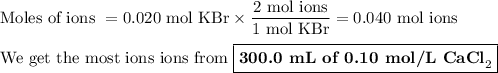Answer:
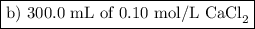
Step-by-step explanation:
a) 400.0 mL of 0.10 M NaCl
(i) Moles of NaCl
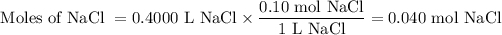
(ii) Moles of ions
NaCl(s) ⟶ Na⁺(aq) + Cl⁻(aq)
We get 2 mol of ions from 1 mol of NaCl
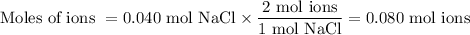
b) 300.0 mL of 0.10 M CaCl₂
(i) Moles of CaCl₂
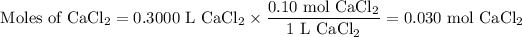
(ii) Moles of ions
CaCl₂(s) ⟶ Ca²⁺(aq) + 2Cl⁻(aq)
We get 3 mol of ions from 1 mol of CaCl₂
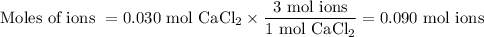
c) 200.0 mL of 0.10 M FeCl₃
(i) Moles of FeCl₃
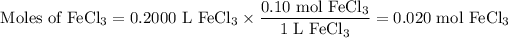
(ii) Moles of ions
FeCl₂(s) ⟶Fe³⁺(aq) + 3Cl⁻(aq)
We get 4 mol of ions from 1 mol of FeCl₃
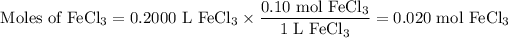
d) 200.0 mL of 0.10 M KBr
(i) Moles of KBr
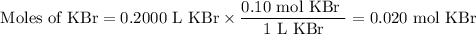
(ii) Moles of ions
KBr(s) ⟶ K⁺(aq) + Br⁻
We get 2 mol of ions from 1 mol of KBr