Answer: The nuclear reaction is written below.
Step-by-step explanation:
Alpha decay is defined as the decay process in which alpha particle is released. In this process, a heavier nuclei decays into a lighter nuclei. The alpha particle released carries a charge of +2 units and a mass of 4 units.
The released alpha particle is also known as helium nucleus.
In this decay process, the atomic number of the atom decreases by 2 units and the mass number decreases by 4 units.
The chemical equation for alpha decay process follows:
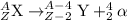
The chemical equation for alpha decay of
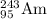 follows:
follows:
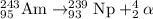
Hence, the nuclear reaction is written above.