Answer:
2 moles of Ferric Oxide (
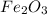 ) over 4 moles of Fe
) over 4 moles of Fe
Step-by-step explanation:
Firts of all I want to explain the nomenclature that is involve in the equation:
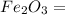 Ferric Oxide
Ferric Oxide
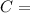 Carbon
Carbon
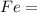 Iron
Iron
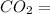 Carbon Dioxide
Carbon Dioxide
Now, to understand the ratio from the balanced equation its necessary read the equation with the respective coefficients, for example:
- 2 moles of Ferric Oxide (
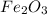 ) reacts to produce 4 moles of Iron (
) reacts to produce 4 moles of Iron (
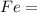 )
) - 3 moles of Carbon (
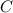 ) reacts to produce 4 moles of Iron (
) reacts to produce 4 moles of Iron (
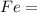 )
) - 2 moles of Ferric Oxide (
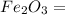 ) reacts to produce 3 moles of Carbon Dioxide (
) reacts to produce 3 moles of Carbon Dioxide (
 )
)
Each of the above could be represent as a ratio, but is always necessary keep the respective coefficient, then if you read each option only the first has the respective coefficient, it means:
2 moles of Ferric Oxide (
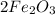 ) reacts to produce 4 moles of Iron (
) reacts to produce 4 moles of Iron (
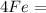 ) or, in other words, 2 moles of Ferric Oxide (
) or, in other words, 2 moles of Ferric Oxide (
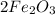 ) over 4 moles of Iron 4 (
) over 4 moles of Iron 4 (
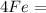 )
)
Regards!