Answer:
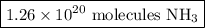
Step-by-step explanation:
We will need a balanced chemical equation with masses, moles, and molar masses.
1. Gather all the information in one place:
M_r: 2.016 17.03
3H₂ + N₂ ⟶ 2NH₃
m/g: 6.33 × 10⁻⁴
2. Calculate the moles of H₂
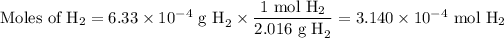
3. Calculate the moles of NH₃
The molar ratio is 2 mol NH₃/3 mol H₂.
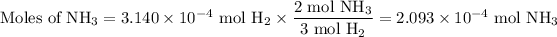
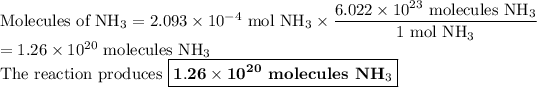
4. Calculate the molecules of NH₃
There are 6.022 × 10²³ molecules of NH₃/1 mol NH₃.