Answer:
Sulfuric acid
Step-by-step explanation:
Acid strength (in water) is measured as the equilibria of dissociation of the acid into hydronium and its conjugated base. The greater the Ka value, greater the acid strength.
This equilibrium constant is denominated Ka
![Ka=([H+])/([HA])](https://img.qammunity.org/2020/formulas/chemistry/college/19d9i4twmz5owvcfuoi0387hvzqc7rimy1.png) where [H+] stands for the molar concentration of hydronium and [HA] stands for the molar concentration of the non-dissociated acid.
where [H+] stands for the molar concentration of hydronium and [HA] stands for the molar concentration of the non-dissociated acid.
The Ka values for these acids are:
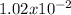 for HClO2;
for HClO2;
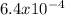 for HF;
for HF;
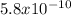 for HBO3;
for HBO3;
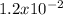 for H2SO3 and
for H2SO3 and
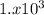 for H2SO4
for H2SO4
So, as the greatest Ka is from H2SO4 it is the strongest acid in this list.