Answer:
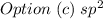
Step-by-step explanation:
Atomic number of boron = 5
In ground state,
Valence shell electronic configuration of B =
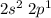
In excited state, one 2s electrons goes into vacant 2p orbital and electronic configuration becomes:
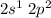
Now, 3 orbitals (2s, and two orbitals of 2p) of B have unpaired electrons. These three orbitals (one 2s, and two orbitals of 2p) undergoes hybridisation to form three hybridised sp2 orbitals.
These 3 hybridised sp2 orbitals are oriented in a trigonal planar arrangement.
The three hybridised sp2 orbitals overlap with 2p orbital of three F to form three B-F bonds.